This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
At Achema, Syntegon showcases its comprehensive expertise in the flexible processing of liquid pharmaceuticals. In addition to an advanced filling machine, an expanded inspection system, process and assembly solutions and comprehensive service offerings will be on display. “Regulations such as Annex 1, high-value drugs, and the increasing use of RTU containers require highly efficient technologies along the entire value chain,” explains Tobias Göttler, Director Product Management Pharma Liquid at Syntegon. “With innovative solutions, extended functions for proven systems, and new services, we enable pharmaceutical manufacturers to produce sensitive drugs according to the most recent requirements of the pharmaceutical industry.”
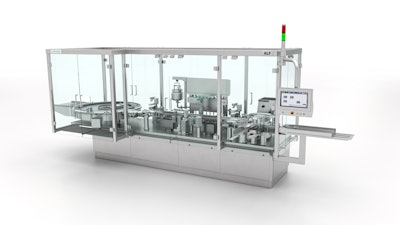
ALF 5000 V: highly precise filling technology
Syntegon demonstrates how this can be achieved with the ALF 5000 V for the precise and gentle filling of small-volume parenterals. “When filling high-priced products such as blood plasma derivatives, the focus is on high yields, minimal product loss, and precise filling processes,” Göttler emphasizes. “The consistent further development of our successful platform fulfills these requirements with new functionalities.” Syntegon’s ALF 5000 V is a flexible solution that enables pharmaceutical manufacturers to combine two filling systems on one platform. While the time-pressure filling system is suitable for large quantities, the peristaltic pump with up to twelve filling stations enables gentle product handling and precise dosing of very small batches. Thanks to the optional twin fill process from the new “Max Pro” portfolio, the filling weight can be optimally determined and readjusted via main and secondary dosing with 100% in-process control at any time.
Hot topic RTU: comprehensive expertise
Ready-to-use (RTU) containers are very popular for both small batches and high-performance lines. Visitors can gain insights into Syntegon’s comprehensive technological and pharmaceutical expertise in the “RTU Open Space” at the booth. In addition to virtual presentations of the Versynta portfolio for small and micro batches, the experts from Syntegon will offer comprehensive advice on all aspects of RTU containers – from Annex 1 compliance and filling with 100% IPC to the use of gloveless barrier technologies.

After filling, the highly precise inspection of products and containers ensures product quality. “With the AIM series, Syntegon has been setting standards in the flexible inspection of vials, syringes, cartridges, and ampoules for decades – as is proven by several hundred machines installed worldwide,” says Göttler. At Achema, the company demonstrates live for the first time how visual inspection and leak detection (CCIT) can be efficiently combined on a space-saving AIM5 for vials. The machine on display features a combination of a pre-rotation tower and camera technology for the visual inspection of liquid and lyophilized products as well as a state-of-the-art measuring sensor in the star wheel for oxygen measurement.

Pens and autoinjectors play a key role in antidiabetics and weight management products. They can be precisely assembled thanks to the new RMA from Syntegon. This space-saving, semi-automatic assembly machine for clinical trials and small batches was developed by Syntegon in close cooperation with customers. The system supports pharmaceutical manufacturers in determining the properties of their drug delivery systems and assembling them in accordance with regulatory standards. The RMA follows Syntegon’s design philosophy on scalability, offering customers a seamless and easy transition to machines with higher outputs when increasing capacity for a market launch.

Compliance with regulatory standards is also the focus of Syntegon’s service offerings. In addition to the successful RABS retrofits for existing equipment, the patented Settle Plate Changer SPC 1000 has recently been added to the portfolio. “Viable monitoring has become even more important when filling sterile products in the context of EU GMP Annex 1” says Steffen Gröber, Global Product Manager Service at Syntegon. “Settle plates may be exposed to cleanroom air for a maximum of four hours and must then be replaced. The new automatic settle plate changer significantly reduces manual operator intervention to replace the plates. This greatly decreases the necessary production interruptions and the negative impact on machine availability.” The SPC 1000, which is available for both existing and new machines, fulfills a further requirement of Annex 1, reducing human intervention in the process zone and the risk of contamination to a minimum.
Comprehensive expertise for liquid pharmaceutical processing
Visitors to the Syntegon booth can also obtain advice on customer and pharma services. These include, for example, service agreements, the cloud-based software solution Synexio, as well as qualification and validation services.
With its broad portfolio, Syntegon covers all steps in the production of liquid pharmaceuticals, including a new bioprocessing system and formulation systems for small-volume parenterals from the subsidiary Pharmatec as well as freeze dryers with a patented loading and unloading concept from Schoeller-Bleckmann Medizintechnik (SBM).
Syntegon will hold the following presentations as part of the congress program in hall 4.1:
- June 11, 3.30 p.m.: From paper to hardware – a technology perspective on EU GMP Annex 1 (2022); Dr. Johannes Rauschnabel
- June 12, 2.30 p.m.: Cell and gene therapies – an equipment builder’s view on current and future challenges; Dr. Andreas Mattern
- June 13, 11.30 a.m.: Towards net zero – carbon emissions from water use in pharmaceutical manufacturing; Christian Lavarreda
Learn more about the technologies and services from Syntegon and meet the experts at Achema, June 10-14, 2024, in Frankfurt at booth C71 in hall 3.1. www.syntegon.com/achema