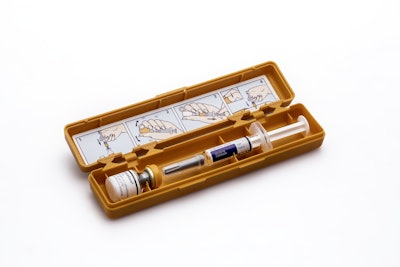
A FiercePharma article dated September 12th discussed a potentially fatal error with a drug injector that treats diabetic patients with severe low blood pressure. Denmark-based Novo Nordisk recalled 6 lots of GlucaGen HypoKit after two customer complaints from the U.K. and Portugal claiming syringes containing sterile water had detached needles.
Only 0.006% of kits have been recalled (about 1 in 17,803) and no adverse incidents have been reported, but patients who do receive the affected injectors are at risk of unconsciousness, seizures, and death. The press release that includes the affected batch numbers was issued on September 8th.