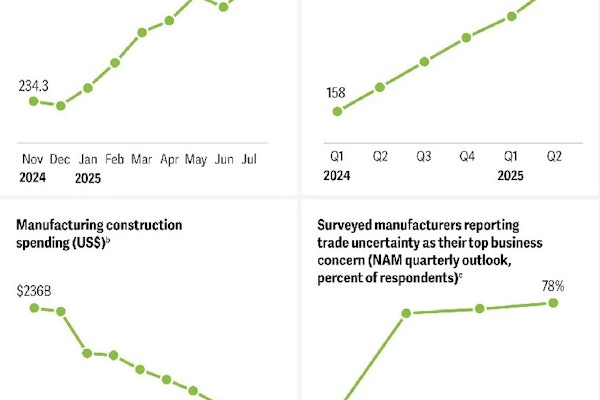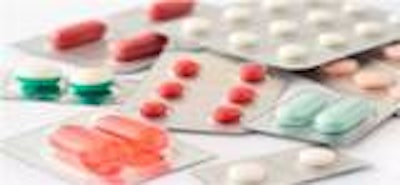
This licence will allow SteriPack to assist companies who require outsourced blister packaging services of tablets and capsules for the pharmaceutical and allied healthcare industries worldwide.
SteriPack's purpose built cGMP compliant Grade D facility is equipped to suit varying requirements allowing flexibility for our customers. Procedures and controls of the facility are in line with the most stringent of requirements but flexible to accommodate customer models as required.
For more information on our new service please Click Here
Companies in this press-release